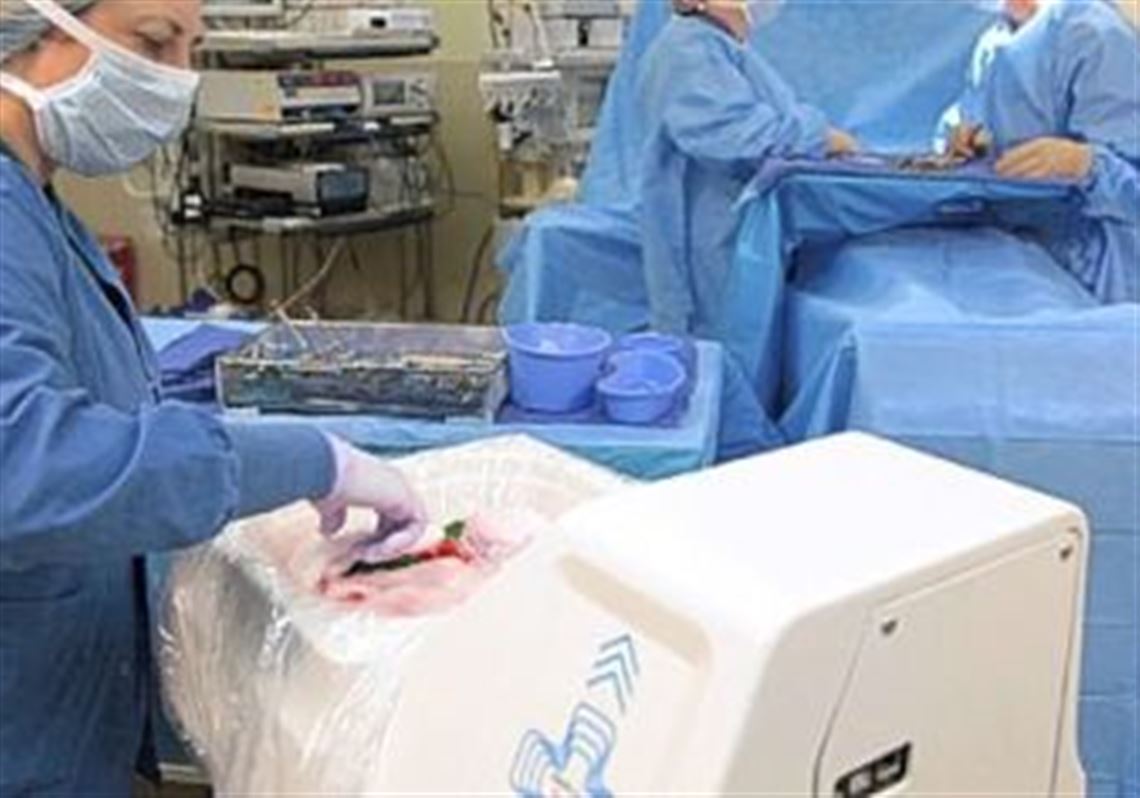
Fresh off an announcement of a new contract with a major California health system, Ross-based ClearCount Medical Solutions today unveiled a new addition to its radio frequency technology system that prevents surgical sponges and towels from getting left inside a patient.
The latest product, SmartSponge Flex, is a special basin for depositing used surgical sponges. It automatically keeps track of a surgical team's sponge count without having to use a wand or a bar code reader.
The new product introduction follows Monday's announcement that the 422-bed University of California, Irvine Medical Center -- the only Level 1 trauma center in Orange County -- has contracted to use ClearCount's technology in its operating rooms.
The hospital is hoping the system will improve patient safety. "We believe that both count reconciliation and detection technology are critical parts of preventing retained surgical sponges," UC Irvine Chief Medical Officer William Barron said in a release. They were also looking for a system with minimal impact on operating room protocols and workflow.
The UC Irvine contract, terms of which were not made public, comes as ClearCount is expanding its reach within the field of medical devices.
Last fall, the company received approval to market its SmartSponge throughout Europe while domestically entering a three-year agreement with Medline Industries Inc. to market its products to more than 2,200 hospitals. The company, founded in 2004, now has its own sales force, and spokesman Jim Sweeney said officials anticipate adding six to 10 new positions in the next 18 months to its current staff of about 20.
Part of the growing demand may be attributed to Medicare's added financial incentive, as the federal program no longer reimburses hospitals for any surgeries needed to retrieve misplaced sponges or towels.
That doesn't happen often -- only about once in every 1,500 open abdominal or open chest operations -- but the medical community calls these incidents "never events" because they should never happen.
And each time a sponge gets left behind, it means at minimum another surgery to remove the item.
For the second consecutive year, the Joint Commission, an independent accrediting organization for hospitals nationwide, has listed "unintended retention of a foreign body" as the most frequently reported unexpected event that risks or causes a patient's serious injury or death.
Based on information collected from hospital reports, the category ranked above patient falls and post-operative complications, with 188 reports in 2011 and 133 in 2010. Because reporting is voluntary, the commission believes the actual number is likely much higher.
The next step for ClearCount is developing surgical instruments that can be tracked. Company officials have said they plan to apply for FDA approval for a line of surgical instruments with radio frequency chips later this year.
"There is a large unmet need in the effective management of surgical instruments," Mr. Sweeney said. "We have made significant research and development progress, and we have overcome a variety of the technical challenges associated with producing a durable and compact form factor suitable for surgical instruments."
First Published: March 21, 2012, 8:00 a.m.